Clinical Electronic Data Capture
Welcome to the Trialomics Electronic Data Capture (EDC) solution showcase. Here you can download a bundle that includes sample documentation, testing plans, and cloud compliance policies used to support two FDA-sponsored clinical trials and the successful launch of Sana’s direct-to-consumer neurotherapeutic product.
Download BundleTrialomics EDC Bundle
Sana Health developed a novel wearable mask designed to help users reduce pain, improve sleep, and elevate mood. The device combines audiovisual neuromodulation techniques to promote calm and relaxation — and it needed rigorous testing to prove its effectiveness.
Trialomics partnered with Sana to support two FDA-sponsored clinical trials evaluating the mask's impact. From the earliest Bluetooth prototypes, our team engineered the complete technology stack — mobile apps, secure cloud infrastructure, and regulatory-ready data systems — to collect and manage study data across participants.
We also managed hosting and compliance for all systems under our cloud platform, Picard, enabling end-to-end oversight of data capture, monitoring, and reporting for the entire study lifecycle.
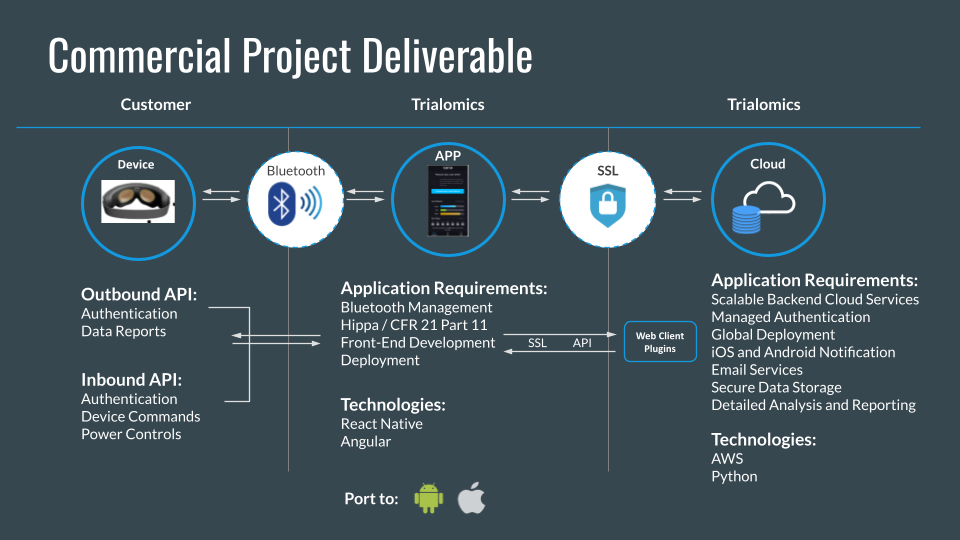
The diagram below illustrates how the wearable mask integrates with the mobile apps and cloud infrastructure used to power the clinical trial.
What’s Inside
The bundle contents demonstrate how we deliver full-cycle clinical data infrastructure, including regulatory-grade systems, patient-facing apps, and secure cloud environments.
Application Documentation
- • Android App User Guide – Instructions for using the clinical-grade mobile application.
- • Sana Android & iOS Screens v4 – UI/UX mockups and final screen references.
- • Sana Clinical Trial Documentation – Overview of trial setup and application workflow.
Validation & Testing
- • Picard Software Validation – System-level testing and validation strategy for Picard.
- • Clinical App & Database Verification – Verification matrix aligned to clinical use cases.
- • Traceability Matrix – Mapping of requirements to test cases for regulatory alignment.
️ Compliance & Hosting (Picard Platform)
The Picard platform is a secure, scalable cloud environment designed for regulated data collection and analysis. This bundle includes:
- • HIPAA & ISO Policies – Security and privacy documents covering encryption, access control, breach notification, and more.
- • Periodic Task Ledger – Audit trail of key compliance and operational tasks.
- • Risk Analysis & Management – Risk assessments and mitigation plans.
Sample Agreements
- • Software and Support Agreement – Example contractual terms covering deployment and support of the EDC infrastructure.
Why This Matters
Clinical EDC requires more than just software. It demands a coordinated system of technology, compliance, and clinical context. Our approach brings these together through:
- • End-to-end control over app development and hosting
- • Regulatory-compliant documentation and traceability
- • Scalable systems that work for clinical trials and commercial products
Click to download bundle.
Download Bundle